Reverse osmosis (RO) technology is widely used in water treatment due to its advantages, such as stable desalination rates, small footprint, automation, and scalability. However, scaling is a troublesome issue for water treatment personnel during membrane operation. Scaling can lead to a decrease in membrane flux, increased energy consumption, lower desalination rates, and a reduced membrane lifespan, which increases operational costs. Therefore, measures must be taken to prevent membrane scaling. Common scaling inhibition methods include two main approaches: adjusting the pH of the RO feedwater and adding scale inhibitors to the feedwater. Both methods can also be used together. This article discusses the scaling inhibition mechanism and provides methods for selecting the inhibition method and calculating the required dosage.
1. Scale Inhibitor Mechanism
Membrane scaling refers to the precipitation of poorly soluble substances, such as CaCO3, CaSO4, BaSO4, and Ca3(PO4)2, on the membrane surface. When these substances become concentrated in the RO system, they can reach supersaturation. For example, at pH=7.5 and water temperature of 25°C, when calcium hardness (measured as CaCO3) is 200 mg/L and total alkalinity (measured as CaCO3) is 150 mg/L, CaCO3 will approach supersaturation. Similarly, at pH=7.5 and water temperature of 25°C, when the concentration of barium ions is only 0.01 mg/L and sulfate ions are 4.5 mg/L, BaSO4 will become supersaturated and precipitate.
The scaling inhibition mechanism of reverse osmosis scale inhibitors primarily involves complexation, dispersion, lattice distortion, and threshold effects.
Complexation and solubilization: Scale inhibitors can form soluble complexes with scaling cations in water, such as calcium, magnesium, and barium ions, preventing the formation of CaCO3, CaSO4, BaSO4, and Ca3(PO4)2.
Coagulation and dispersion: The anions released by the scale inhibitors attach to CaCO3 crystals. Since contaminants in industrial wastewater typically carry a negative charge, like charges repel each other, creating electrostatic repulsion that prevents CaCO3 crystals from aggregating and growing into larger particles. The crystals are dispersed uniformly in the solution, thereby inhibiting the formation of CaCO3 scales.
Lattice distortion: During the aggregation and growth of CaCO3 microcrystals, scale inhibitors are incorporated into the crystal lattice or at the crystal interface, causing lattice distortion. This directly inhibits or distorts crystal growth. For example, CaCO3 is formed by positively charged calcium ions and negatively charged bicarbonate ions, which grow in a specific direction. During their development, scale inhibitors are incorporated into the lattice, increasing internal stress within the crystal. When the stress reaches a certain threshold, the crystal will rupture, preventing crystal formation.
Threshold effect: The scale inhibitors disrupt the aggregation and ordering processes of CaCO3, CaSO4, BaSO4, Ca3(PO4)2 microcrystals, thus preventing precipitation.
2. Selection of Scaling Inhibition Methods
The primary indicator used to evaluate the risk of scaling in reverse osmosis (RO) systems is the Langelier Saturation Index (LSI). When LSI < 0, the water has no tendency to scale (although it may be slightly corrosive). When LSI ≥ 0, the water is prone to scaling. The pH adjustment method prevents scaling by lowering the pH of the feedwater, thus shifting the LSI from greater than 0 to less than 0. Adding scale inhibitors can prevent scaling even when LSI ≥ 0, as insoluble microcrystals in the water cannot grow, aggregate, or precipitate. The main mechanisms for this inhibition are the four described above. Currently, domestic scale inhibitor products can ensure that insoluble substances do not precipitate even when LSI = 3. International top-brand inhibitors can guarantee no precipitation at LSI = 5. However, it’s important to be cautious when purchasing inhibitors, as some domestic vendors import concentrated international brand inhibitors and dilute them with large amounts of water, leading to significant discrepancies in the actual scaling inhibition performance, even though the product is labeled as LSI = 5.
1. pH Adjustment Method
To ensure the production of qualified permeate water, RO feedwater pH is typically controlled between 6 and 9, with some companies implementing more refined control within a narrower range, such as 7.0 to 8.5. Extremely low or high pH levels in the feedwater can prevent the RO permeate from meeting the required water quality standards. Therefore, the pH adjustment method for scaling inhibition assumes that the RO permeate pH will be within the desired range. It is important to note that the pH adjustment method primarily targets CaCO3 scaling and is ineffective against other types of scaling substances.
2. Scale Inhibitor Addition Method
As mentioned earlier, adding scale inhibitors can allow RO membranes to tolerate higher LSI values. However, RO scale inhibitors tend to be expensive, with domestic products ranging from 0.008 to 0.012 RMB/g and international top-brand concentrated products costing between 0.055 and 0.075 RMB/g, resulting in high operating costs.
Additionally, there are numerous types of scale inhibitors on the market, and some manufacturers constantly promote new, unproven concepts, leading to confusion when selecting a scale inhibitor. Generally, mature commercial scale inhibitors can be classified into three categories: phosphorus-based scale inhibitors, polymer-based scale inhibitors, and environmentally friendly scale inhibitors.
-
Phosphorus-based Scale Inhibitors: These include inorganic phosphate inhibitors (such as sodium tripolyphosphate or sodium hexametaphosphate) and organic phosphonate inhibitors (such as hydroxyethylidene diphosphonic acid, amino-trimethylenephosphonic acid, and phosphonic acid derivatives). Inorganic phosphate inhibitors contain long-chain anions and are prone to hydrolysis, especially at higher temperatures. When hydrolyzed, they form phosphoric acid salts, which can react with calcium ions to form Ca3(PO4)2, a scale with a lower solubility product than CaCO3. Therefore, inorganic phosphate inhibitors are unsuitable for water with high temperatures or high calcium ion concentrations.
-
Organic Phosphonate Scale Inhibitors: These inhibitors contain organic phosphonates, typically characterized by the C-O-P bond. When exposed to high temperatures and alkaline environments, organic phosphonates can hydrolyze into phosphoric esters and alcohols, significantly reducing their scaling inhibition efficiency. Consequently, organic phosphonates are not suitable for use in water with high temperatures or high pH values.
Polymer-based scale inhibitors are primarily divided into anionic and cationic polymer inhibitors. The former is mainly used to prevent metal ion scaling, while the latter is primarily used to inhibit silica scaling. The main ingredients in polymer-based inhibitors are acrylic acid and maleic acid, and during formulation, various functional groups are introduced into the molecules. As a result, polymer scale inhibitors come in various formulations. When using these inhibitors, it is important to consider not only the water quality conditions but also the types of scales present. For example, polymer inhibitors with carboxyl groups primarily target calcium scaling, sulfonic acid-based polymer inhibitors are mainly used for metal oxide scaling, and amine-based polymer inhibitors are effective for silica scaling. Therefore, polymer scale inhibitors are not broad-spectrum agents; they are designed to address the shortcomings of broad-spectrum inhibitors. Additionally, since the primary component of polymer-based inhibitors is a polymer, they are susceptible to oxidation by chlorine and other oxidative biocides, which can render them ineffective. Therefore, before adding these inhibitors, it is necessary to first neutralize any residual chlorine in the water by adding a reducing agent.
Environmental scale inhibitors typically contain active ingredients such as polyaspartic acid, polyepoxysuccinic acid, and their derivatives. These inhibitors are mainly used to address calcium-based scales like CaCO3, CaSO4, and CaF2. The advantage of these inhibitors is that they can tolerate relatively high calcium ion concentrations. For instance, even when the calcium ion concentration reaches 500 mg/L, they can still achieve over 80% inhibition of calcium scaling. However, these inhibitors require higher dosages, cause significant changes to the water's pH, and are less effective at temperatures below 40°C. Since the maximum allowable feedwater temperature for reverse osmosis membranes is 35-40°C, these inhibitors are generally not suitable for use in reverse osmosis systems but are more commonly used in cooling water systems.
3. Dosage Calculation
As mentioned earlier, whether the water is prone to scaling depends on the Langelier Saturation Index (LSI) value. Therefore, whether using acid dosing to adjust the pH or adding scale inhibitors to prevent reverse osmosis membrane scaling, the essence is controlling the LSI of the water. The calculation of LSI is as follows:
In the formula:
- pH is the measured pH value of the reverse osmosis concentrate.
- pH_s is the saturation pH value corresponding to the carbonate system in the water at the actual water temperature, known as the saturation pH.
The pH of the reverse osmosis concentrate can be easily obtained through online instruments or manual measurement. Therefore, the key to calculating LSI is determining pH_s. According to the Standard Methods for Examination of Water and Wastewater, pH_s can be calculated using the following formula.
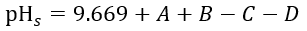
In the formula:
- A is the total dissolved solids (TDS) coefficient.
- B is the water temperature coefficient.
- C is the calcium hardness coefficient.
- D is the total alkalinity coefficient.
The calculation methods for A, B, C, and D are as follows.
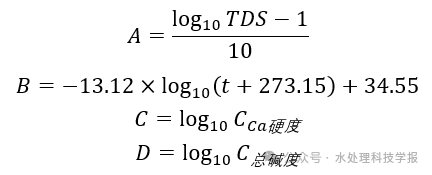
- TDS is the total dissolved solids content in the reverse osmosis concentrate, in mg/L.
- t is the temperature of the reverse osmosis concentrate, in °C.
- Cca is the calcium hardness of the reverse osmosis concentrate, expressed as CaCO3, in mg/L.
- C_total alkalinity is the total alkalinity of the reverse osmosis concentrate, expressed as CaCO3, in mg/L.
Using the example mentioned earlier, where pH = 7.5, TDS = 2000 mg/L, temperature t = 25°C, calcium hardness Cca = 200 mg/L, and total alkalinity C_total alkalinity = 150 mg/L, the process of calculating the LSI is as follows:
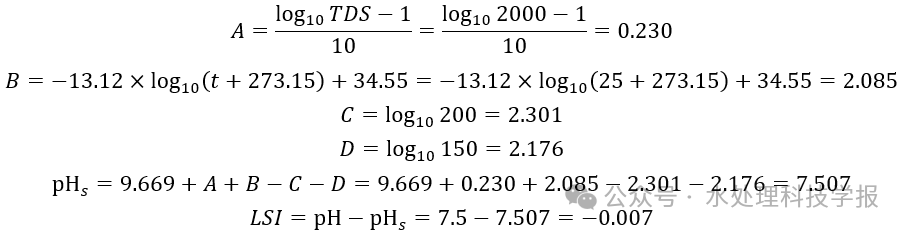
This aligns with the previous statement, where under these conditions, CaCO3 is nearly saturated. Furthermore, we can observe that the dosage calculation can be expressed by the following three formulas.
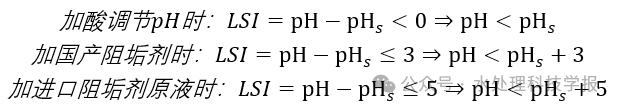
The specific application method is as follows:
First, we measure the TDS, temperature t, calcium hardness Cca, and total alkalinity C_total alkalinity of the reverse osmosis concentrate. Then, using the formula, we calculate pH_s.
- If pH_s ≥ pH, no further adjustments or scale inhibitors are needed to prevent calcium scaling.
- If pH_s < pH, we ensure that after adjusting the pH, the reverse osmosis feed water pH does not drop below 6.5 (since a lower pH may result in acidic reverse osmosis product water). In this case, we can adjust the pH by adding acid until pH_s ≥ pH. This is only applicable when pH_s ≥ 6.5. If pH_s < 6.5, we need to adjust the pH with acid until it reaches 6.5 or even lower, which will cause the reverse osmosis product water to become acidic.
- If pH_s < 6.5, scale inhibitors must be added.
It is important to note that, as mentioned earlier, acid dosing for pH adjustment primarily targets CaCO3 scaling and is ineffective for other types of scaling. For other scaling substances, a scale inhibitor is required for control.
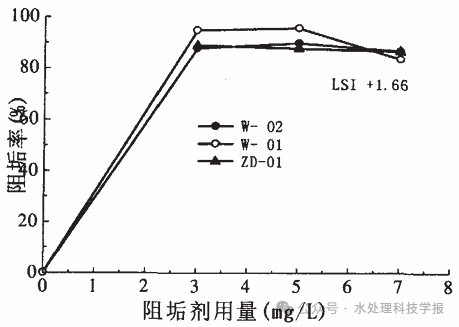
For the acid dosing to adjust pH, the dosage can be controlled through the actual measured pH. As for the scale inhibitor dosage, extensive research by domestic and international scholars has shown that:
- When the scale inhibitor dosage is below 2.5 g/m³, the inhibition efficiency is relatively low.
- When the dosage exceeds 3.0 g/m³, the inhibition efficiency no longer improves significantly.
Thus, the optimal dosage of scale inhibitor is between 2.5-3.0 g/m³, as shown in the following chart.
In summary, when preventing reverse osmosis membrane scaling, we should first calculate the LSI of the reverse osmosis concentrate using the formulas provided in this article to assess whether scaling is likely to occur. Secondly, we need to analyze the main scaling substances in the permeate, which can be determined by testing indicators such as Ca²⁺, Mg²⁺, HCO₃⁻, Ba²⁺, SiO₂, etc. This analysis allows us to make targeted decisions on whether to adjust the pH with acid or to add scale inhibitors. If a scale inhibitor is required, we should determine the appropriate type and dosage of the inhibitor to use.
 En
En
 عربى
عربى 中文简体
中文简体

